VaxxCellence can support this by offering the following services:
Supported by
Designing the early preclinical and clinical phases with a view on who will be treated and why – asking the right questions
Starting at the bench and driven by the scientific concepts, it is critical to define both the research and the development objectives in a forward-looking way. This means that early evidence generation should support and inform the entire series of next steps in the CDP.
VaxxCellence supports this process for a range of potential products, such as:
Readings:
A scientific concept is connected to a medical need and the type of product to address that need. ‘First time in human’ (FTIH) is a key milestone that is the next step after the Discovery phase.
Readings:

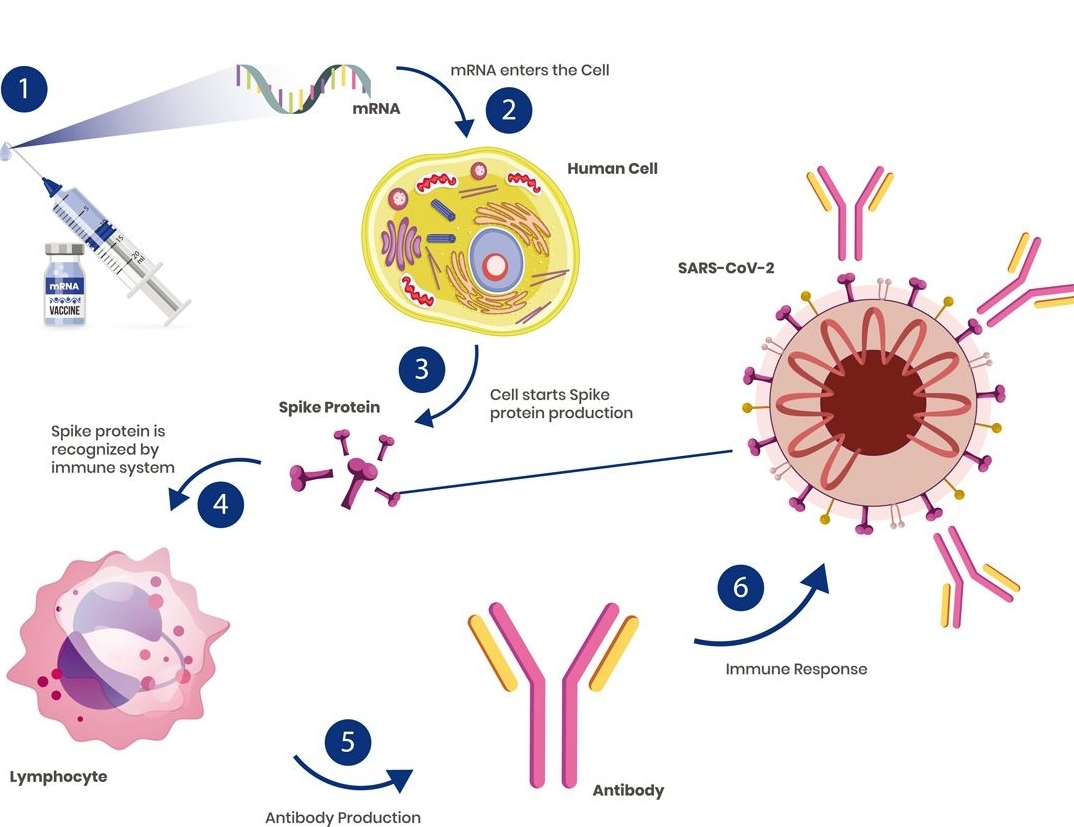
Understanding the mode of action is important for multiple reasons. First, it guides the clinical readout strategy and any immunological endpoints. Second, it provides the scientific framework required to interpret clinical data, ranging from immunological results to reactogenicity, safety and efficacy data. Third, it opens the door to iterative product improvements. As an example, the mode of action of an mRNA/LNP vaccine is illustrated. VaxxCellence can support key steps such as:
Readings: